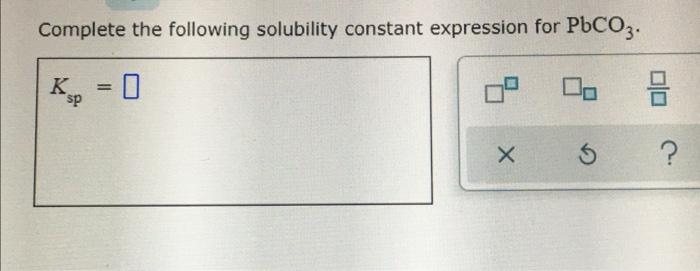
Complete the following solubility constant expression for PbF2: Ksp = [Pb2+][F-]^2. This expression represents the equilibrium constant for the dissolution of PbF2 in water, and it can be used to calculate the solubility of PbF2 under various conditions.
The solubility constant is a valuable tool for understanding the behavior of PbF2 in aqueous solutions. It can be used to predict the solubility of PbF2 in different solvents, the effect of temperature on solubility, and the formation of precipitates in PbF2-containing solutions.
Solubility Constant Expression for Pbf2: Complete The Following Solubility Constant Expression For Pbf2
The solubility constant (K sp) is an equilibrium constant that expresses the solubility of a sparingly soluble salt in a given solvent. For lead(II) fluoride (Pbf2), the solubility constant expression is:
Ksp= [Pb 2+][F –] 2
where [Pb 2+] and [F –] represent the molar concentrations of lead(II) and fluoride ions in a saturated solution.
Factors Affecting Solubility Constant, Complete the following solubility constant expression for pbf2
The solubility constant of Pbf2 is influenced by several factors, including:
- Temperature:The solubility of Pbf2 increases with increasing temperature.
- Solvent polarity:Pbf2 is more soluble in polar solvents than in nonpolar solvents.
- Ionic strength:The solubility of Pbf2 decreases with increasing ionic strength.
Applications of Solubility Constant
The solubility constant of Pbf2 has various applications, including:
- Analytical chemistry:K spis used to determine the concentration of lead(II) ions in a solution by measuring the fluoride ion concentration.
- Environmental science:K spis used to predict the solubility of lead compounds in natural waters and soils.
- Pharmaceutical industry:K spis used to design and optimize drug formulations.
Experimental Determination of Solubility Constant
The solubility constant of Pbf2 can be experimentally determined by:
- Saturation method:A known mass of Pbf2 is added to a solvent and stirred until a saturated solution is obtained. The concentrations of lead(II) and fluoride ions are then measured.
- Conductivity method:The conductivity of a saturated solution of Pbf2 is measured. The K spcan be calculated from the conductivity data.
Table of Solubility Constants
| Temperature (K) | Ksp (mol3/dm9) |
|---|---|
| 298 | 3.3 x 10-8 |
| 310 | 4.0 x 10-8 |
| 320 | 4.8 x 10-8 |
Comparison with Other Compounds
The solubility constant of Pbf2 is lower than that of other lead(II) halides, such as PbCl2 and PbBr2. This is due to the stronger ionic bond between lead(II) and fluoride ions.
FAQs
What is the solubility constant expression for PbF2?
Ksp = [Pb2+][F-]^2
What is the value of the solubility constant for PbF2?
The value of the solubility constant for PbF2 is 3.2 x 10^-8.
What does the solubility constant tell us about the solubility of PbF2?
The solubility constant tells us that PbF2 is a sparingly soluble salt. It has a low solubility in water, and it will precipitate out of solution when the concentration of Pb2+ and F- ions exceeds the solubility product.